Everyone one of us is likely aware of what lead — as in the metal — is. Having a somewhat dull, metallic gray appearance, it occupies atomic number 82 in the periodic table and is among the most dense materials known to humankind. Lead’s low melting point and malleability even when at room temperature has made it a popular metal since humans first began to melt it out of ore in the Near East at around 7,000 BC in the Neolithic period.
Although lead’s toxicity to humans has been known since at least the 2nd century BC and was acknowledged as a public health hazard in the late 19th century, the use of lead skyrocketed in the first half of the 20th century. Lead saw use as a gasoline additive beginning in the 1920s, and the US didn’t abolish lead-based paint until 1978, nearly 70 years after France, Belgium and Austria banned it.
With the rise of consumer electronics, the use of lead-based solder became ever more a part of daily life during the second part of the 20th century, until an increase in regulations aimed at reducing lead in the environment. This came along with the World Health Organization’s fairly recent acknowledgment that there is truly no safe limit for lead in the human body.
In this article I’ll examine the question of why we are still using lead, and if we truly must, then how we can use this metal in the safest way possible.
The Miracle Metal

An interesting detail about lead is that it doesn’t just have one stable isotope, but that it has a total of four stable forms, with three of these being the most common. These are 206Pb, 207Pb, and 208Pb, which result from the nuclear decay of 238U, 235U and 232Th respectively. 204Pb is the primordial form, meaning that it was created along with the other materials that ended up forming this planet and does not result from any decay chain. This makes 204Pb useful for radio dating rocks by comparing its ratio with the other (radiogenic origin) stable lead isotopes.
Lead is easily obtained from galena (lead(II) sulfide), requiring only relatively low temperatures to extract the lead. The use of lead as an everyday metal developed throughout the prehistoric into the classical era, with the Ancient Egyptians being the first to use it in applications like cosmetics, a practice that would later spread to Ancient Greece and into post-medieval Western societies. The Ancient Romans would use lead extensively for water pipes (the English word ‘plumbing’ being derived from the Latin word for lead (plumbum), where its resistance to corrosion was highly praised.

Lead saw significant use as a writing material, in the form of lead tablets, as well as for slingshot bullets. It was also part of the wine and food preparation process, as sweeteners and preservatives that were prepared in a lead(-lined) vessel would impart a pleasant sweet taste to the food and wine on account of the lead(II) acetate (‘sugar of lead’) that was formed, compared to the bitter verdigris one would get with copper or bronze vessels.
Starting in the 11th century, European architecture used lead extensively for roofing and piping, also evolving into its use in stained glass. The invention of the printing press meant a key role for lead, as did the invention of (practical) firearms. Lead was an essential component in whitening cosmetic (Venetian ceruse), which were heavily used by Western European aristocracy and royalty, as well as by Japanese geishas starting in the 18th century.
Lead Exposure Routes
Europe’s Industrial Revolution saw lead production for the first time exceed that of Ancient Rome, with lead being extensively used for plumbing, paints and lead-acid batteries. This was also the era when physicians began to strongly link exposure to lead with a variety of mental and physical disorders associated with ingesting lead. The UK would enact its first laws aimed at reducing lead exposure in factories in the 1870s.
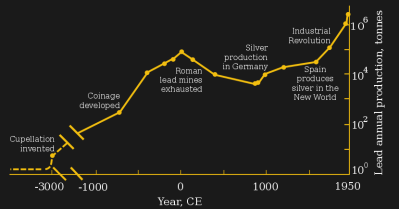
By this time it was primarily occupational exposure to lead that formed the main risk: people mining lead ore and those involved in smelting and otherwise processing the metal would breathe in the lead dust and vapors, and ingest lead (by licking one’s lips, etc.). Absorption through the skin is less common, but as in the case of lead-based cosmetics can happen in the form of long-term exposure to low (but constant) amounts of elemental lead.
The frustrating thing is that humanity had previously discovered and seemingly forgotten the dangers of lead exposure. During the 2nd and 1st century BC Ancient Greek and Roman scientists and physicians noted the effects of lead on the human body, describing symptoms such as gout, mental degradation, colic and paralysis. While the Middle Ages saw a general ignorance of the dangers of lead, the centuries after it saw a sharp rise in the awareness of the dangers of lead exposure.
Lead is a Dangerous Mimic of Calcium
Lead dust can easily enter the bloodstream, with nearly half of the lead dust one breathes ending up in the lungs, of which almost all gets absorbed into the blood where it binds to erythrocytic and plasmatic proteins. From there it is distributed to the remaining tissues of the body. At this point the cellular mechanisms that underlie lead’s neurotoxicity and general toxicity take hold. The half-life of lead in the blood is about 35 days, while it can stay for 2 years in the brain and many years in the bones, providing a long-term reservoir that becomes especially risky in case of extreme long-term physical exertion, such as a pregnancy.
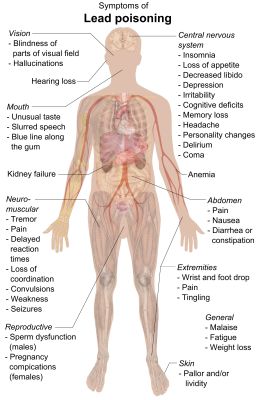
The main risk in lead exposure lies in how it interacts with the body’s biochemical processes. Because of its chemical composition, it can take the place of calcium in the body, as well as that of zinc and magnesium. Because of this it gets easily incorporated into virtually every tissue of the body, where its mismatch with an actual calcium, zinc or magnesium molecule proceeds to cause enzymes to fail and stop functioning. Worse is that lead has a larger ionic radius than zinc and calcium, meaning that it more readily binds to enzymes than the latter two.
After binding to an enzyme, this biochemical disruption can impair a wide variety of processes. The most dangerous of this is probably lead’s interference with the delta-aminolevulinic acid dehydratase (ALAD) enzyme, which is crucial for the biosynthesis of heme, the cofactor in hemoglobin. If lead levels are high enough, this leads to anemia, as well as damage to neurons due to build-up of aminolevulinic acid. In addition, lead causes DNA damage and destroys a cell’s structures because of disruption to critical cell processes.
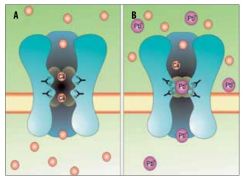
Because of this same mimicry of calcium, lead is able to easily pass through the blood-brain barrier via the calcium-ATPase pumps of the cells that form the barrier, the same way lead easily enters other cells. Here too, its biochemical differences with calcium causes it to disrupt the biochemical processes it interacts with, causing disruption of synapse formation, neurochemical (neurotransmitter) development, loss of ion channels, and generally stunts the development and overall functioning of the brain.
Lead ions (Pb2+) function as NMDA receptor blockers, being biochemically similar to Ca2+ ions, but end up blocking the receptor’s channel pore, effectively disabling it. Unlike Mg2+ ions, the Pb2+ions aren’t easily dislodged. Depending on the amount of lead ions in the brain, the resulting effect can be mild to crippling. Combined with the other neurotoxic and cytotoxic effects of lead, severe brain damage is to be expected.
Lead in the 21st Century
Although lead-based paint and lead in gasoline are mostly a thing of the past now, environmental contamination of lead is still very much present. As a heavy metal, it doesn’t decay, meaning that the lead pollution from industrial and other processes — such as energy production from coal — is still around, with lead contamination especially in cities is quite profound. Any exposed lead will end up in the environment, via (rain) water or as dust, making it an ongoing issue.

Like humans, plants readily absorb the lead in the environment, where it can end up in our food and that of other animals whom we consume later. The inhalation of radioactive 210Pb (and subsequently 210Po through decay) as a component of cigarette smoke from these components which the tobacco plant absorbed while it was growing is still a major issue, although here the main problem is mostly caused through these alpha particles and not the toxicity of the lead itself.
In civilized nations at least, workplace safety has improved to the point where occupational exposure is minimal, and significant lead exposure for the average person is unlikely. Contributing to this has been the work of countless researchers during the 20th century who have made the case for lead toxicity, resulting in the World Health Organization (WHO) along with the US CDC reducing the ‘acceptable’ limit from about 50 µg/dL in the 1980s to <5 µg/dL today, with no safe limit.
Efforts are underway to ban lead as a component for bullets, as these cause lead contamination of the environment. Any lead that remains in an animal after it has been shot can end up being consumed by humans as well as predators and the like. Alternatives for lead are steel, tungsten-nickel-iron, bismuth-tin, and tungsten-polymer.
Lead at Home
Barring existing contamination such as leaded paint in the US, lead contamination at home is often self-introduced, such as when handling lead in some form during construction work, creating or handling lead fish sinkers, lead bullets and similar (including casting). The use of lead-containing solder alloy is also still a common practice and likely touches each person reading this article. For all of these scenarios the same rules apply:
- Do not eat or drink at the working area.
- Do not put lead (or lead solder) in your mouth.
- Do not breathe in the fumes.
- Do not touch your face while handling lead.
- Clean your hands with soap after handling lead.
- Wet clean any surfaces that may be contaminated with lead dust
This is especially crucial at home where children and pregnant women are more likely to be present, due to the disastrous effect that lead’s neurotoxicity has on the developing brain and other tissues of a child and fetus.
Times Change
Back in 1983, the US Navy commissioned a study (PDF) to examine just how pervasive lead contamination was in an environment where lead-based solder was being used for soldering work. Because of the generous WHO limits back then, it was considered not a real issue assuming one took the above precautions into account. When compared against today’s WHO recommendations, the same precautions still apply, but 36 years later the recorded amounts of lead dust would be significant cause for concern.
One thing about heavy metals is that, like radiation, you cannot see it even when it’s there. The lead dust flaking off and coating surfaces, equipment and your own hands is invisible, and unless it’s in a form your tongue can detect (like lead(II) acetate), you’re unlikely to notice that you just ingested it. Nor will you taste the lead in vegetables, rice, or that piece of venison. The effects of lead-poisoning can take years to become apparent, by which time it’ll be too late.
The advantage we have today is that after thousands of years of experience with this heavy metal, thousands of observations and courtesy of modern sciences a good understanding of why exactly it is that lead is so dangerous to all life on this planet. Because of this, we can still keep making use of its blessings while taking further measures to prevent it from harming us and the environment.
No comments:
Post a Comment