Current global events have demonstrated that we do not live in the most stable of times. Still, most of us 90’s kids are probably glad that we did not have to endure the political shakiness of the Cold War era when people were living in constant fear of nuclear Armageddon. Nuclear weapons tests were common during this period as the United States and the Soviet Union invested heavily to increase the quality and quantity of their warheads in the race for nuclear supremacy.
Even though the political situation stabilized after the fall of the Soviet Union, the consequences of the vast amount of nuclear tests conducted back then are still noticeable today. Besides the devastating effects on human health and the environment, this period also leaves some implications for science which are not always negative.
A Not So Brief History of Nuclear Testing
Credit: CT24BTO
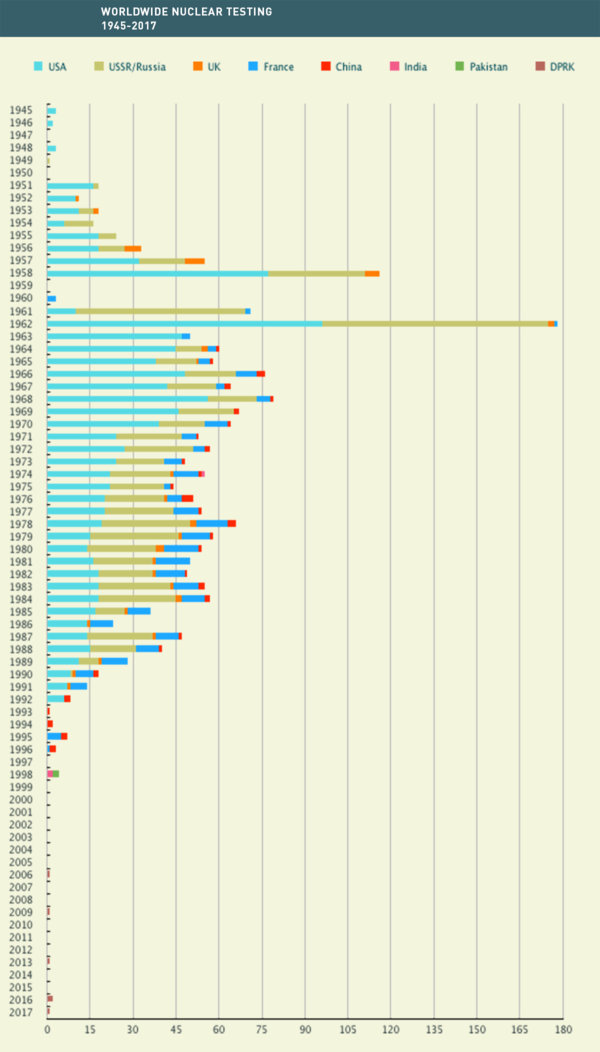
The first nuclear test was famously conducted under the codename Trinity by the United States in July 1945 as part of the Manhattan Project. The second country to follow was the Soviet Union in 1949, then the UK in 1952, France in 1960, and China in 1964. This was followed by countries like India, Pakistan, and most recently North Korea. In total over 2,100 nuclear tests have been carried out until today with an estimated total yield of about 540 megatons. The majority of these tests were carried out by the Soviet Union and the United States during the Cold War with as many as 178 nuclear explosions in the year 1962 alone.
In 1963, after having exploded hundreds of nuclear bombs above ground for the past 18 years, politicians finally concluded that they were done poisoning the atmosphere with radioactivity and ratified the Partial Test Ban Treaty (PTBT). Since then, all forms of nuclear tests were prohibited except for those conducted underground. This underground testing was only banned in 1996 by the Comprehensive Nuclear-Test-Ban Treaty (CTBT), which finally prohibits all nuclear explosions on Earth.
A nuclear explosion produces a vast amount of radioactive isotopes. These are either direct fission products or originate from the activation of other materials by thermal neutrons. In atmospheric tests, the radioactive material is propelled into the upper atmosphere before it comes down as nuclear fallout. The table below shows some of the radionuclides produced by nuclear testing that have an implication for scientific research.
| Isotope | Half-life | Origin |
|---|---|---|
| Co-60 | 5 years | activation |
| Kr-85 | 10.8 years | fission |
| Sr-90 | 29 years | fission |
| Cs-137 | 30 years | fission |
| C-14 | 5,700 years | activation |
Bomb Pulse Dating
Credit: Hokanomono
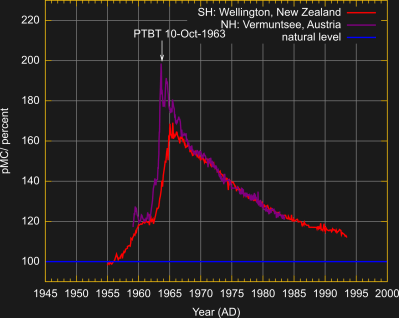
An interesting example of a radionuclide is C-14 which is also naturally produced in the atmosphere when neutrons generated by cosmic rays get absorbed by nitrogen atoms. The C-14 then combines with oxygen to form radioactive CO2.
Since a nuclear explosion floods the atmosphere with thermal neutrons it also generates C-14 through the same mechanism. As this figure shows, the C-14 concentration in the northern hemisphere was almost doubled by nuclear testing until the PTBT was signed. Luckily, the artificially produced C-14 poses no health risk and has a useful application in radiocarbon dating. Classical radiocarbon dating only works for very old organic materials since it is built on the principle that the C-14/C-12 ratio slowly decreases according to the C-14 half-life after an organism has died. Bomb pulse dating instead looks at biological samples from the nuclear testing era and compares their amount of C-14 with the decreasing atmospheric abundance of the bomb pulse. With this method, it has been possible to determine the age of unidentified bodies and study the regeneration of neuronal cells.
Detecting Fake Wine with Cs-137
Credit: CENBG
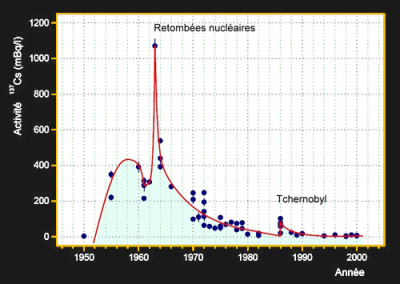
A similar dating method exists for Cs-137, an isotope that poses one of the greatest health risks of all fallout products due to its long half-life and chemical properties. Cs-137 is almost purely man-made and can be found in trace amounts in soil, plants, and animals all around the world where it has been distributed by nuclear weapons and accidents. As Cs-137 emits gamma-rays that can penetrate closed containers it can be detected non-destructively and has been used to identify the authenticity of wine bottles. Obviously, a wine which has been bottled before 1945 should not contain any Cs-137. Older wines can be dated by comparing their activity with the reference curve shown on the left.
Man-made Radioactivity Obscures Dark Matter Signal
The radioisotopes generated by nuclear testing did not all benefit Science. For some fields in physics, like the search for dark matter, the detection of neutrinos, or the search for rare nuclear decays, the unwanted background radiation from our legacy of nuclear testing gets in the way.
The XENON-1T/nT experiment, for example, is aiming at the detection of dark matter using a giant detector filled with liquid xenon. Commercially available xenon contains trace amounts of krypton at the ppm level and therefore also the fission product Kr-85. As the signal from the beta decay of Kr-85 interferes with the potential dark matter signal, the xenon first has to be purified to sub ppt levels with a special two-story-high distillation column. Another pesty background for rare event searches comes from Co-60 present in post-World War II steel. Before a detector is built one usually conducts extensive screening campaigns to identify steel with low Co-60 contamination, know as low background steel.
Nuclear Explosions Increase Rainfall
Researchers recently showed that nuclear weapon tests also had a significant influence on the distribution of rainfall. For their study, they were looking at weather data between 1962-64 from research stations in Scotland and the UK. It was shown that the Sr-90 released into the atmosphere during nuclear weapons tests significantly increased the air conductivity through ionization. The excess charge encouraged the formation of raindrops and lead to visibly thicker clouds and an average increase in rainfall of 24% on days with higher radioactivity. These findings help to better understand the role of electric charge in cloud formation with possible future applications in geoengineering.
The period of heavy nuclear testing certainly left their mark on our world. Naturally, we should not put scientists again in the situation where they try to find the silver lining in having the earth radioactively contaminated.
No comments:
Post a Comment