Lithium ion batteries have been a revolutionary technology. Their high energy and power density has made the electric car a practical reality, enabled grid storage for renewable energy, and put powerful computers in the palm of the hand. However, if there’s one thing humanity is known for, it’s always wanting more.
Potential contenders for the title of ultimate battery technology are out there, but it will take a major shift to dethrone lithium-ion from the top of the tree.
Dominant For Good Reason
Lithium-ion batteries were first developed by Stanley Whittingham, working at Exxon, who were looking to diversify away from oil in the midst of the major energy crises of the 1970s. Over the years, the technology was developed further, with work by John Goodenough (a superb hacker name if we’ve ever heard one) and Akira Yoshino increasing performance with improved cathode and anode materials. Commercialization was first achieved by Keizaburo Tozawa, working at Sony to develop a better battery for the company’s line of camcorders.

The lithium-ion rechargeable battery has the ideal attributes for portable power, with high energy density and high power density, This means that it can store plenty of energy, and release it quickly for applications that draw lots of current. Low weight and high power output were game changing — technologies where size and weight matter, like quadcopter drones and powerful smartphones simply, wouldn’t be practical with older, heavier battery technologies.
In the years since, the world has fallen in love with lithium batteries. Different chemistries abound, optimising the batteries for more recharge cycles, higher power outputs, or lower cost. Saying production has skyrocketed in recent years is a bit of an understatement, with the coming of age of portable consumer electronics and the electric car revolution running almost entirely on rechargable lithium battery technology.
However, challenges remain. Electric cars are still somewhat range limited compared to their gasoline counterparts, and recharge times further frustrate the issue. Huge gains have been made in recent years, but automakers continue to strive for better performance as a competitive advantage. Additionally, while prices have dropped precipitously in the last ten years, lithium batteries still aren’t exactly cheap. Compounding this is a reliance on minerals that can be scarce or difficult to source. This has been particularly true of cobalt, leading some manufacturers to explore alternative lithium-ion chemistries, and we’re beginning to see success in completely removing cobalt from the equation.
There remains scope for alternative technologies to challenge lithium’s dominance of the battery industry. Any contender will need great energy density and power density, as well as the ability to last for thousands of charge cycles. Additionally, low cost, ease of manufacture, and being less prone to catastrophic failure, are all targets that battery researchers are trying to reach.
On the Cusp of Greatness?
The road from the lab to the factory is a long one, and many exciting projects run into intractable engineering issues long before reaching commercial sale. Breakthroughs are exactly that, the point at which hard problems are suddenly solved, so let’s look at what’s potentially on the cusp of greatness:
Solid State for Lithium Metal and Better Safety
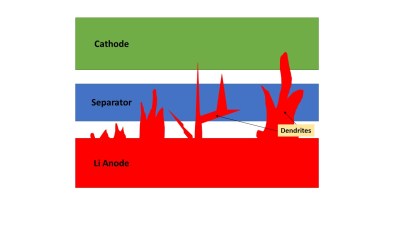
Lithium-ion batteries use liquid electrolytes of a variety of chemistries to tweak performance characteristics for particular applications. However, replacing this liquid with a solid state electrolyte is a hot research topic, as it promises a multitude of gains over current batteries.
Many hold the solid-state electrolyte as the solution that will enable the use of lithium metal anodes in batteries, replacing graphite in most current applications. Typically, lithium metal is an unstable anode material due to dendritic growth caused by chemical reactions with the liquid electrolyte. If a solid state electrolyte eliminated this problem, batteries could use the better performing lithium metal anode material. This would enable a huge gain in energy density, up to 2.5 times greater than conventional lithium-ion batteries.
The liquid electrolyte is also to blame for a lot of the danger inherent in lithium-ion batteries, becoming highly flammable, or even explosive, when a battery undergoes a thermal runaway condition. Solid-state batteries may also solve this problem, with reduced flammability compared to traditional liquid electrolytes.
There are still roadblocks in the way of full-scale uptake of solid state battery technology. Conductivity issues at room temperatures continue to hamper the technology. Manufacturing challenges also exist, with many designs requiring the use of vacuum deposition techniques. Regardless, many companies are pouring money into solid-state battery research, with Samsung in particular working hard to develop the technology.
Lithium Air is Chasing the Energy Density of Gasoline
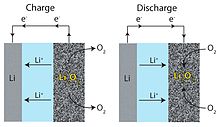
Lithium-air batteries work by using oxygen in the atmosphere as a reactant. Oxygen donates electrons to the lithium via a carbon cathode. Theoretically, such cells could have a specific energy of 11,680 Wh/kg, close to that of gasoline at 13,000 Wh/kg — far exceeding contemporary battery technologies. Excluding the mass of oxygen, energy density per mass is up to 10 times higher than lithium-ion, meaning the technology would be ideal for increasing the range of electric vehicles.
A multitude of challenges face the lithium-air battery before it can successfully be commercialized. Chemical stability has been a problem of early efforts. Further development of cathode materials continues to bear fruit, but best-case results from lab testing have cells lasting just two months in practice. Charging efficiency is also low — just 65% of the energy put in during charging is usable. Additionally, the requirement for gaseous oxygen as a reactant poses further problems. While lab tests can use purified oxygen, atmospheric air contains carbon dioxide, water vapor, and other contaminants that can damage the battery. These would need to be filtered out in practical designs.
Flow Batteries
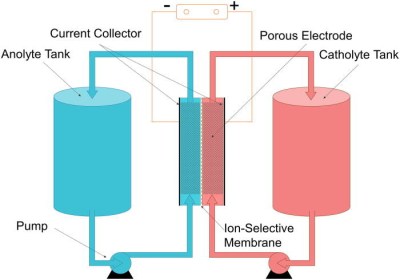
Flow batteries are a concept involving two liquids which are pumped through a membrane, exchanging ions and generating electricity in the process — you may remember reading Kristina Panos’ article on liquid air energy storage just a few weeks ago where the technology is being considered for grid storage.
As flow batteries rely on charge stored in liquid form, bigger batteries can be created by simply building bigger tanks for the reactants. The battery can be quickly “recharged” by simply replacing the electrolyte, or alternatively, it can be regenerated electrically like a traditional rechargeable battery. The easy scaling makes the technology appealing for grid storage, while the potential to be able to quickly “refuel” the battery would solve the problem of recharging electric vehicles quickly.
Despite their benefits, flow batteries have some drawbacks which have held them back from any serious use. Storing and pumping liquids is far more mechanically complex than traditional batteries, which can typically be treated as solid lumps of matter that may just require a little cooling now and then. This complexity and extra equipment reduces power density and makes flow batteries less practical for transport applications. Most research focuses on energy storage for home and grid-level applications instead.
Conclusion
It may yet be some time before we see major change in battery technology in most of our devices. It’s likely that solid-state batteries that still rely on lithium chemistries will be the main contender that will overhaul lithium-ion’s dominance in the transport sector, with potential to follow in devices like laptops and smartphones. Lithium-air and flow batteries have further hurdles to overcome before they reach viability.
However, development continues on existing lithium-ion technology by academics and industry all over the world, particularly due to the demand in the automotive sector. With capacity and performance improving each year, we may yet continue to see classic lithium-ion remain as the battery of choice for quite some time to come.
No comments:
Post a Comment